Nickel Deposits Explained
Magmatic Nickel Sulphide Deposits
The bulk of nickel mined today comes from either nickel laterite deposits, in deeply weathered terrains where nickel is released from the weathering of ultramafic rocks, or from nickel sulphide deposits which is our primary focus at Grasset. In Canada nickel sulphide deposits are typically found in clusters or “belts” often spanning 10’s to 100’s of kilometres. These include deposits in the Voisey’s Bay area of Labrador, the Raglan (Cape Smith) belt of northern Quebec, the Thompson belt in northern Manitoba and a number of deposits in the Timmins area in the southern Abitibi. The well-known nickel deposits of the Sudbury basin, while sharing a number of features in common with these other deposits, are believed to be related to ultramafic activity triggered by a meteorite impact and are thus in a class of their own.
The Model
Nickel is believed to be a primary component of the earth’s core and largely concentrated in the core and mantle. In the near surface it is most commonly found in association with ultramafic (or mafic) rocks which are high temperature, iron-magnesium rich, typically intrusive rocks sourced from the upper mantle or very deep crustal levels. Current modeling suggests the ultramafic magma’s rose toward the surface along the kind of mantle plumes – or hot spots – which produce island arc chains like the still forming Hawaiian Islands.
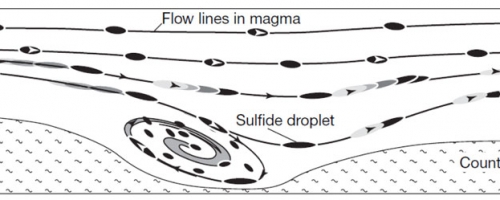
The model below, from Barnes and Lightfoot (2005), outlines current thinking which relates to the formation of nickel-copper-PGE sulphide deposits similar to what Balmoral has discovered at Grasset.
Within the ultramafic intrusion or flow, sulphide droplets form, often through contamination of the magma with sulphur from adjacent rock units, like the pyritic volcanic and sedimentary rocks surrounding the ultramafic complex at Grasset. These sulphide droplets are convected through the magma along flow lines as shown below. As they convect through the magma they collect or scavenge nickel, copper and the platinum group elements from the magma – as all of these elements have a strong chemical affinity for sulphur. As the sulphide droplets accumulate metals they become heavier than the magma itself and begin to sink through the magma and accumulate in depressions in the base of the ultramafic (see (e) above).
Sulphide Textures – A Key to Recognizing and Navigating in Magmatic Nickel Systems
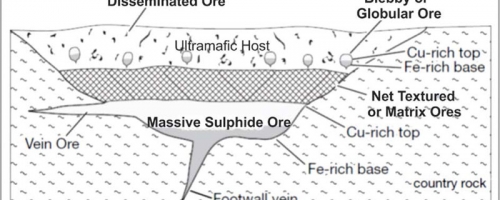
Ultimately sufficient sulphides will accumulate within these depressions to form nickel-copper-PGE orebodies. These orebodies are characterized by a number of distinct textural elements (Figure 3 below). Already at Grasset we have seen most of the elements characteristic of an idealized magmatic nickel-copper-PGE sulphide system.
Working from top to bottom of the system we see at the highest levels broad zones of disseminated (or interstitial) sulphide mineralization. You can think of these as individual sulphide drops frozen in place within the magma – sulphides that either didn’t have the time to sink before the magma crystallized or drops that didn’t reach sufficient size to sink. Typically this type of Disseminated Ore is seen above and lateral to the higher grade, more massive parts of the system. One of the characteristics of magmatic sulphides is that the individual sulphide grains – like the orebodies as a whole – tend to be zoned having a more copper-rich top and nickel rich base. Thus magmatic sulphide grains are typically multi-phase being comprised of separate chalcopyrite (copper-rich), pyrrhotite (iron-rich) and pentlandite (nickel-rich) phases.
A number of open pit nickel deposits are developed within these disseminated zones which tend to be more laterally extensive than the massive sulphide zones. Often nickel systems progress no further than this disseminated phase. The large Dumont nickel deposit, located 200 kilometres south of Grasset near Amos, Quebec would be an example of a large, disseminated nickel deposit which lacks appreciable semi-massive or massive sulphide zones. Drill hole GR-14-16 displayed over 65 metres of disseminated nickel bearing sulphides.
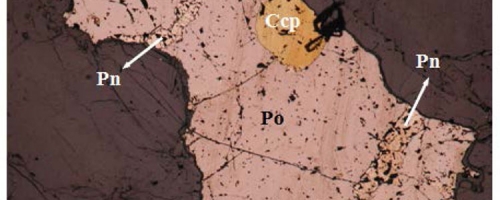
Deeper into the systems the sulphide drops begin to coalesce as they start to sink to from what is known as “Blebby” or “Globular” Ore. These “blebs” may reach several centimetres in size and range from aggregates of droplets to semi-massive sulphide “balls”. This type of texture is relatively rare, as the blebs are effectively caught in place as they falling through the magma. The photo below is from Grasset hole GR-14-22 showing classic “blebby” or globular sulphides, note the disseminated sulphide grains around the blebs. Blebs comprised mainly of pyrrhotite with lesser pentlandite and chalcopyrite in ultramafic (peridotite) matrix.
As the sulphides continue to sink we see net-textured (or matrix) ores which are the most common ore type in most high-grade nickel deposits. Here sulphides range from 5 to as much as 50+% of the rock, forming a matrix between silicate minerals. Depending on the dynamics of the magma chamber the sulphides can be thought to have sunk between and cemented together earlier formed silicate minerals or the silicates may have settled into a sulphide pool as the chamber cooled. The genesis can be argued either way but what we end up with is a “net” of partially connected sulphide grains. In some cases there is enough connectivity between the sulphide grains for them to produce weak to moderate geophysical (EM or electromagnetic) conductors. All of the mineralization styles above will typically produce I.P. (induced polarization) anomalies. As recently reported by Balmoral drill hole GR-14-25 displayed over 40 metres of net textured sulphides as show in the photo below.
Ultimately, at the base of the sequence, the sulphide grains will settle until they dominate the base of the depression and form massive nickel-rich sulphides. These are typically the richest parts of any magmatic nickel system but massive nickel sulphide bodies are surprisingly rare, suggesting most systems crystallize before allowing the time for, or don’t have the flow dynamics or geometry to generate, formation of massive sulphides. The presence of massive nickel sulphides in our third hole at Grasset (GR-14-17) was thus very encouraging. Typically the more massive parts of the system are moderately to highly conductive as we have seen at Grasset.
Structural Modification
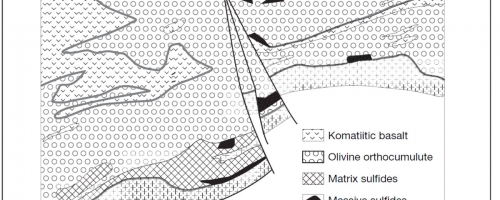
Following the formation of a nickel sulphide zone subsequent activity can modify these original textures. In many cases subsequent magma pulses into the host intrusion, or even new ultramafic volcanic flows, can partially or completely erode the early formed sulphide zones. In some cases, as in the Raglan area of northern Quebec, subsequent magma pulses can lead to the formation of multiple “stacked” nickel zones within the host intrusive sequence as shown in the diagram below. Early evidence at Grasset indicates potential for a similar scenario with nickel mineralization being observed at multiple levels within the Grasset ultramafic complex. In drill hole GR-14-25 a massive sulphide zone at the top of the net-textured mineralization would appear to relate to a second magma pulse, later than the one forming the net textured mineralization, which has cut down into and incorporate some of the net-textured mineralization at its base forming a massive sulphide layer.
Subsequent deformation, after the formation of the nickel ore bodies, can have a variety of effects and modify primary magmatic textures in a variety of ways. In the Thompson nickel camp of northern Manitoba many of the better orebodies have been remobilized into regional fold noses and have steeply plunging morphologies more similar to Archean gold deposits than classic nickel sulphide deposits.
How Do I Compare This to Balmoral’s Gold Assets
It is always difficult to compare the potential value of different deposit types, in particular when multiple metals are involved. To provide a relative sense of how say a 10 g/t gold intercept compares to a 1% nickel intercept we often resort to a gold or nickel equivalent calculation. There are several assumptions built into these types of calculations which mean they are far from perfect comparisons and as they are typically based on metal prices they are a snap shot in time. They also assume 100% metal recoveries which clearly make them “non-real world” as no deposits exhibit 100% recovery of actual assay values.
At current metal prices (April 29, 2014) of $8.30 per lbs for nickel and $1300 per ounce gold a rock containing 1% nickel, on a 100% recovered basis, would be equal in terms of recovered value to a rock containing 4.38 g/t gold on the same 100% recovered basis. Obviously most nickel deposits contain significant copper, platinum, palladium or other recoverable minerals which then ultimately add to the potential value of the intercept. For example, again on a 100% recoverable basis adding 0.10% copper (at $3.20 copper), 0.15 g/t platinum (at $1400/ounce platinum) and 0.35 g/t palladium (at $800/ounce palladium) to the same rock adds a potential value of 0.53 g/t gold equivalent to this same rock (for 4.91 g/t gold equivalent). These numbers serve only as a guideline to assist in making a comparison and should not be relied on in making any form of investment decision.
For the calculations above:
The gold grade equivalent used is as follows: Gold Equiv. (g/t) = (Ni grade x ((Ni price per lb/Au price per ounce) x 0.0686 lbs per oz x 10000 g per %)) + (Cu grade x ((Cu price per lb/Au price per ounce) x 0.0686 lbs per oz x 10000 g per %)) + (Pt grade x (Pt price per oz/Au price per oz)) + (Pd grade x (Pd price per oz/Au price per oz)).
The metal prices used were: Gold $1300/oz, Nickel, $8.30/lbs, Copper $3.00/lbs, Platinum $1400/oz, Palladium $800/oz.
References:
Barnes, Sarah-Jane and Lightfoot, Peter C. 2005; Formation of Magmatic Nickel Sulphide Deposits And Processes Affecting Their Copper and Platinum Group Element Contents. Society of Economic Geologists 100th Anniversary Volume, pp. 179-213.